We discuss effects of the war in the Israeli market, UK CBD regulatory changes, the future of the French medical scheme, and the legislative process in Germany.
Canada celebrates 5 years of legalisation amidst sector consolidation, while momentum in the Australian and UK medical markets perseveres, and the Dutch recreational experiment readies for launch later this year.
Studies on the ‘entourage effect‘ support greater effects of full-spectrum cannabis and highlight that aroma is influenced by minor compounds other than terpenes.
We hope you enjoy this month’s cannabis brief!
1. Regulatory updates:
The outbreak of the war in Israel has significantly affected the cannabis industry: employees have been killed or kidnapped by Hamas, while pharmacies experience personnel shortages due to military conscription and the reluctance of Arab workers to leave their neighbourhoods:
- Displaced patients have had their prescriptions extended, while deliveries to different addresses are now allowed.
- Spike in prescriptions for trauma has been met by the Ministry of Health with a warning that cannabis treatment is only to be used as a last resort.
The UK government has recently permitted electronic prescriptions of cannabis medicines, which can significantly ease prescribing process and speed up patient access in the growing market:
- The Food Standards Agency has updated its consumer advice for CBD setting a max recommended daily dose of 10mg of CBD, while accepting a recommended THC limit.
- Holland & Barrett pulls 31 CBD products from shelves in response to FSA guidance.
- Industry commentators criticise the toxicology study conducted by a consortium led by EIHA, which led to the slashing of the recommended dose of CBD.
In France, medical cannabis will finally be included in the social security budget for 2024 while the current experimentation with under 3,000 patients will likely be extended for 9 months:
- €10M have been allocated for the transition period before generalisation of the scheme expected from the beginning of 2025.
- Medical cannabis products will require authorisation on a case-by-case basis for 5 years.
- Discussion is ongoing regarding the ‘last resort’ status of medical cannabis, the extent of reimbursement, and the inclusion of inhaled forms of medical cannabis (dried flower).
Legislative process of the cannabis bill including clubs and personal cultivation is ongoing in Germany, but second phase consisting of commercial pilot projects is in jeopardy:
- Conservative opposition dominant in the German Senate remains against legalisation plans and would likely veto it. Opinion polls show that the current government could lose its majority in the 2025 elections, casting doubts on its ability to pass legislation.
- The launch of vape cartridges in the German medical market has caused controversy as the association of cannabis dispensing pharmacies claims no approved medical devices with the appropriate CE certification exist.
- German pharmacies face difficulties with reimbursement of dronabinol.
- The consideration of CBD oils as either food or medicine is being discussed with different courts ruling in favour of classification as medicine, ruling out the applicability of novel food regulations.
Federal cannabis reform in the United States is gaining bipartisan support after a new proposal by republican representatives would establish a federal licensing system, regulate interstate commerce and implement a 3% federal cannabis excise tax.
- Georgia becomes the first state to allow pharmacies to dispense medical cannabis. On top of 7 licensed dispensaries, 120 pharmacies will supply cannabis to patients with a doctor’s approval.
- Despite most states having already decriminalised cannabis, a report on the State of Cannabis Justice shows shortcomings in record clearance and resentencing policies of many states.
- Fake cannabis labor unions expand foothold across the United States.
The roll-out of adult-use pilot projects continues in Switzerland, while federal authorities move to ban the latest synthetic cannabinoids (HHCP, THCP, H4CBD…):
- Health regulator has greenlighted a pilot project in Basel-country participated by German company Sanity Group. “Grashaus” dispensaries will open to service up to 3,950 users.
- Lausanne launches its “Cann-L” experiment with up to 1,200 users.
- In Geneva a “cannabinotheque” will open in December supplying up to 1,000 volunteers.
Supply gaps in the Italian medical cannabis market continue 8 years after the military took control of domestic production due to inefficacious State intervention:
- New imported medical cannabis extracts produced by Tilray are approved for reimbursement in the Lombardy and Emilia Romagna regions.
- The recent decree classifying CBD as a narcotic has been suspended by an Administrative Court until January.
New Zealand will allow sales of medicinal CBD without a prescription. CBD medicines that obtain approval will be considered a pharmacist-only medicine in doses of under 150mg a day.
The European Directory for the Quality of Medicines has pre-published the Cannabis flower monograph ahead of its publication in the European Pharmacopoeia expected in January 2024.
Malta has granted the first 2 licenses to cannabis user associations allowing them to start growing and selling cannabis legally.
The South African government opens a new public consultation of the Cannabis for Private Purposes Bill with the intention to decriminalise cannabis cultivation and use.
In Morocco, the national cannabis agency tasked with overseeing legal cultivation of cannabis has appointed a general director.
Brazilian state of Espiritu Santo approves the supply of CBD medicines free-of-charge.
Fiji eyes medicinal cannabis cultivation to diversify tourism-focused economy.
Infographic of the month: 5 years of Canadian legalisation
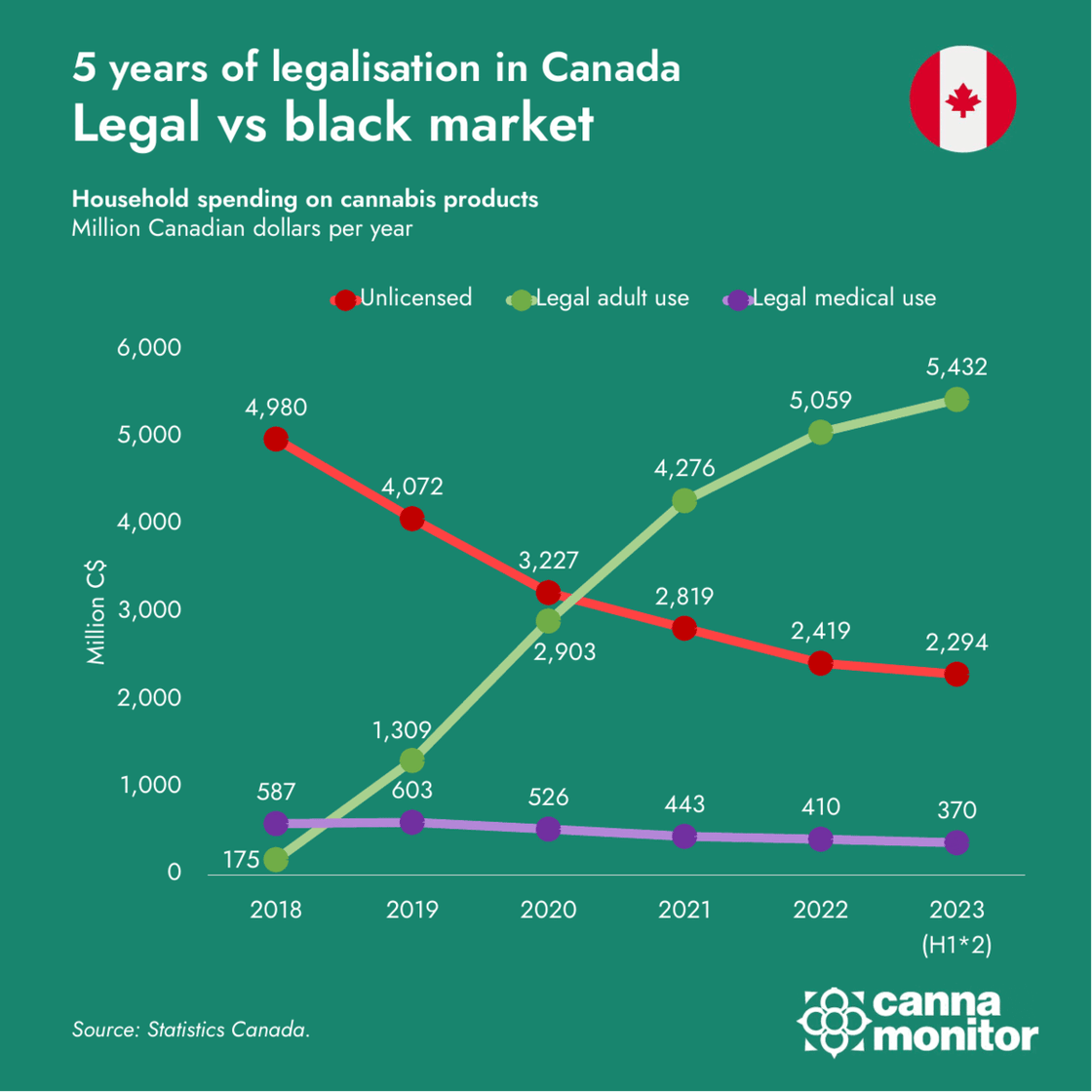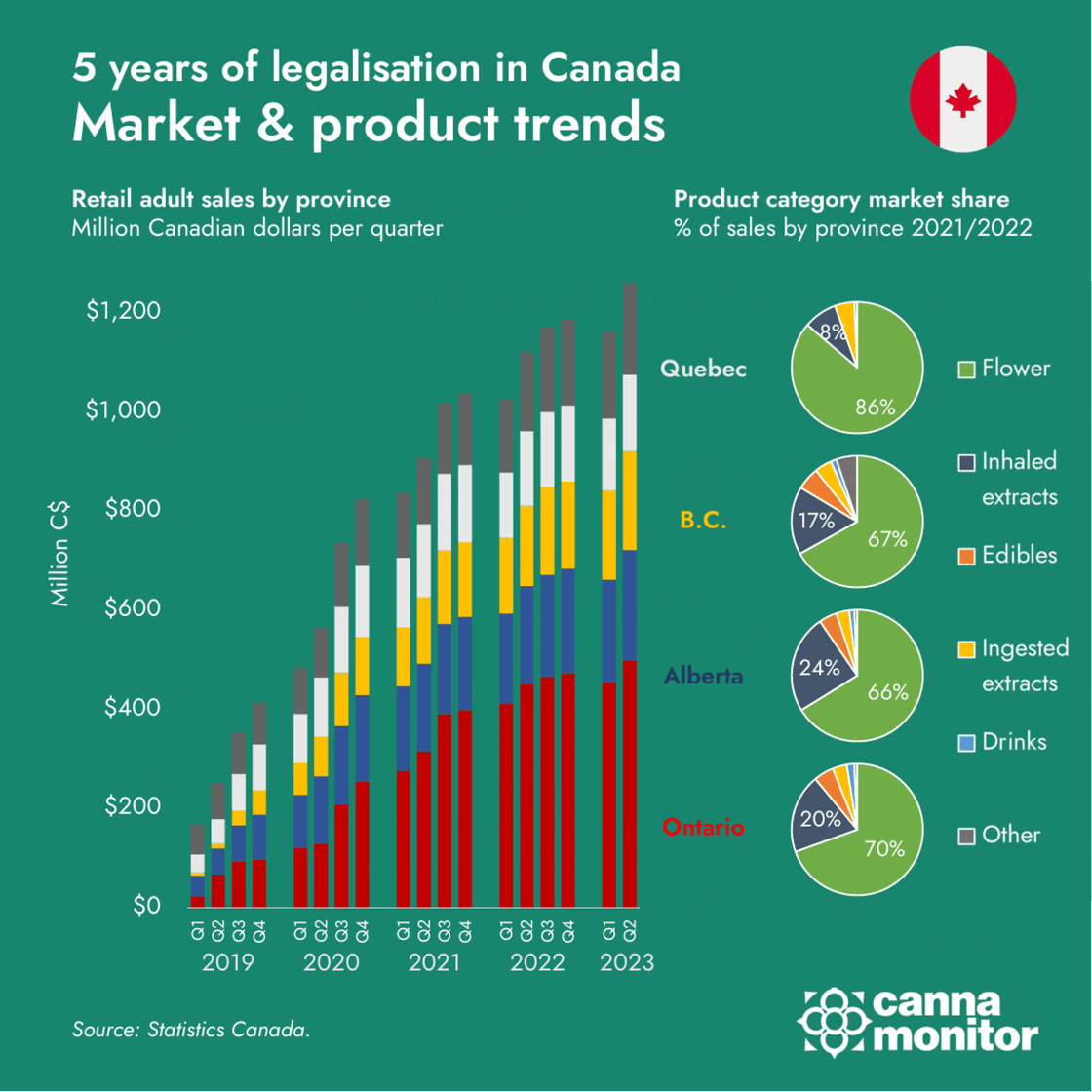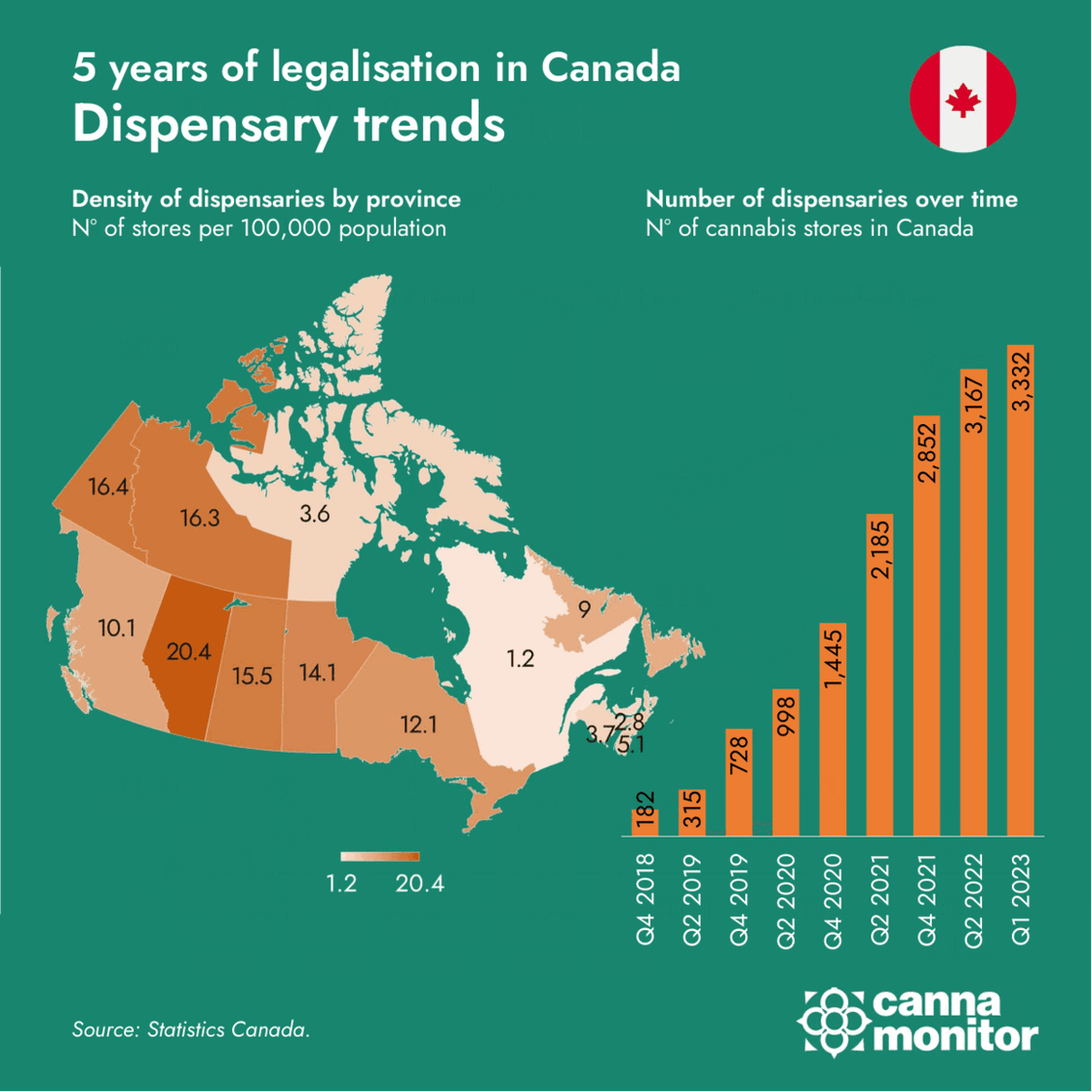
2. Market and Company Updates:
Canada celebrates 5 years of legalisation with legal sales taking over 70% of the market, while pre-rolls continue to increase market share and now represent 1/3 of all legal sales. However, sector consolidation continues with further layoffs and diversification to adjacent categories despite the positive trend of medical cannabis exports:
- Canopy Growth’s drinks subisidary BioSteel files for creditor’s protection, as its Kincardine facility achieves EU GMP certification by German inspectors.
- SNDL implements $10M cost-savings with the closure of Alberta flagship facility.
- Tilray diversifies into beer and fruits and vegetables as its cannabis business falters.
- MediPharm Labs settles $9M lawsuit with Tilray and signs new agreement as preferred supplier.
- Aurora settles patent dispute with Willow Biosciences over biosynthesis of cannabinoids.
The positive momentum of the Australian medical market perseveres with 304,000 patient approvals through authorised prescribers in the first half of the year, more than double the amount last year. However, there are concerns regarding the prescription of high-THC products for anxiety.
- ECS Botanics signs A$24M flower supply deal with MediCann Health.
- Bioxyne subsidiary imports A$3.2M of cannabis flower from the UK.
- Ab Initio will manufacture dronabinol for ResolutionRx to develop its sleep apnea drug.
- Vitura acquires telehealth platform Doctors on Demand for A$25M.
- Releaf expands clinics and dispensary network with a 13th location.
- SyqeAir Inhaler has been included in the ARTG through partner Novachem.
- CBD supplier Endoca Australia rebrands as Aura Therapeutics.
In the United Kingdom 24 tonnes of medical cannabis have been imported this year, a 300% year on year increase over the 8 tonnes imported last year, as domestic growers get ready to commence commercial operations.
- Grow Pharma inks deal with Midlands-based grower Dalgety to bring UK-grown cannabis medicines to market by the end of 2023.
- Sydney-based Cannim acquires UK CBD retailer and distributor Savage Cabbage.
- Canadian Psilobrain Therapeutics snaps Vitality CBD brand from Yooma Wellness for U$2M.
- British Cannabis™ acquires CBD brand Hempura.
The Netherlands gets ready for the launch of its recreational supply chain experiment later this year:
- Holigram, one of the 10 growers selected for the controlled supply chain project, joins forces with Paradise Seeds as its genetics supplier for the experiment.
- Kenzoll, a private equity investor of the experiment grower Hollandse Hoogtes, acquires majority ownership in medical cannabis cultivation facility Luxacan in Zimbabwe.
- Progress in cultivation facilities is underway with Q-farms beginning construction of its facility and Arista BV obtaining municipal approval.
Germany braces ahead of limited legalisation next year:
- Ilios Santé (Bloomwell) launches the first high-THC cartridges for vaporisation in the German market under the ‘Breezy’ brand, despite concerns over the legality of the required devices.
- Storz & Bickel launches new flower vaporiser ‘Venty’, its first new product in almost a decade.
- Cannamedical inks collaboration with telehealth provider Algea Care (Bloomwell).
- Demecan launches topical cream with THC and CBD to treat dermatological diseases.
- Cantourage signs yet another supply deal with Canadian craft producer VASCO.
- Danish Stenocare brings extracts to the German medical market through ADREXPharma.
Canndoc launches 11 new flower strains in Israel despite delays caused by the war, while Israeli tobacco importer GlobrandS enters cannabis space with acquisition of distribution assets.
In Switzerland, Astrasana launches high-THC medical cannabis flowers in collaboration with German distributor Cantourage.
Pharmaceutical company Neuraxpharm expands collaboration with Israeli cannabinoids producer Panaxia to launch products in the Swiss and Czech markets.
In Ireland, Sativex is now available for reimbursement through the publicly funded healthcare system for multiple sclerosis patients. A high-THC oil by Althea has been approved for reimbursement for CINV patients, after its balanced formulation received approval in July.
Portugal has exported 5.4 tonnes of cannabis in the first half of 2023, primarily to Germany, Poland and Australia. Blossom Pharma has obtained EU-GMP Part I and Part II for cannabis manufacturing.
New flower varieties have been launched to the Polish medical market, including Headband by Tilray, Bienville by ODI pharma, and Shishkaberry and Gorilla Glue by CanPoland.
Luxembourg risks another medical cannabis shortage as Danish Schroll Medical substitutes Tilray as the main supplier after winning a public tender.
Colorado has reached $15 billion in legal cannabis sales since it legalised in 2014. However, low prices and oversupply have caused a fall in revenues in recent quarters.
California cannabis operators file lawsuit against Cookies for the sale of hemp-derived intoxicating cannabinoids such as Delta-8 THC.
Medical cannabis pilot program in Denmark recovers its growth after the reintroduction of full-spectrum oils.
3. Science updates
Novel electroencephalogram study demonstrates faster onset of full spectrum cannabis compared to isolates, suggesting the ‘entourage effect’ is responsible for psychoactive effects over THC %.
- Aroma differences of cannabis are driven by minor compounds different than terpenes, finds study.
A UCLA dentistry-led team has received a $5M federal grant to explore the use of synthetic cannabinoids in managing pain associated with oral cancer.
Avextra has announced the initiation of ‘Belcanto’, a phase II clinical trial with 170 oncology patients in palliative care that will receive a 10THC/10CBD extract.
Tilray supports a Spanish clinical trial on the use of medical cannabis for Glioblastoma.
Study in the Netherlands confirms overall satisfaction among medical cannabis patients.
Australian BOD Science inks deal with UK-based Brains Bioceutical to supply API for its CBD-based insomnia medication planned to be launched as an OTC in Australia.
- Two studies in the US evaluated the effects of CBN, with and without CBD, on sleep.
Hemp as a cover crop in New Zealand vineyards found to have benefits such as enhanced soil health, improved crop yield, and additional income for farmers.
$2 million research project in Cornell University hopes to develop hemp varieties for U.S. latitudes.
- USDA approves genetically engineered hemp with super low levels of THC.
Brazil is preparing to establish its first genetic bank of cannabis.

