Revamping of regulations, mixed news for the CBD sector and launches of new medical cannabis products.
August has seen a revamping of cannabis regulations in Israel and Argentina, and positive signs for the federal status of cannabis in the US and Germany.
Israel will legalise over-the-counter CBD, while Italy, Portugal and Brazil tighten up rules for CBD market operators.
Competition in established medical cannabis markets heats up with the launch of new products, and large medical studies are underway.
1. Revamping of regulations:
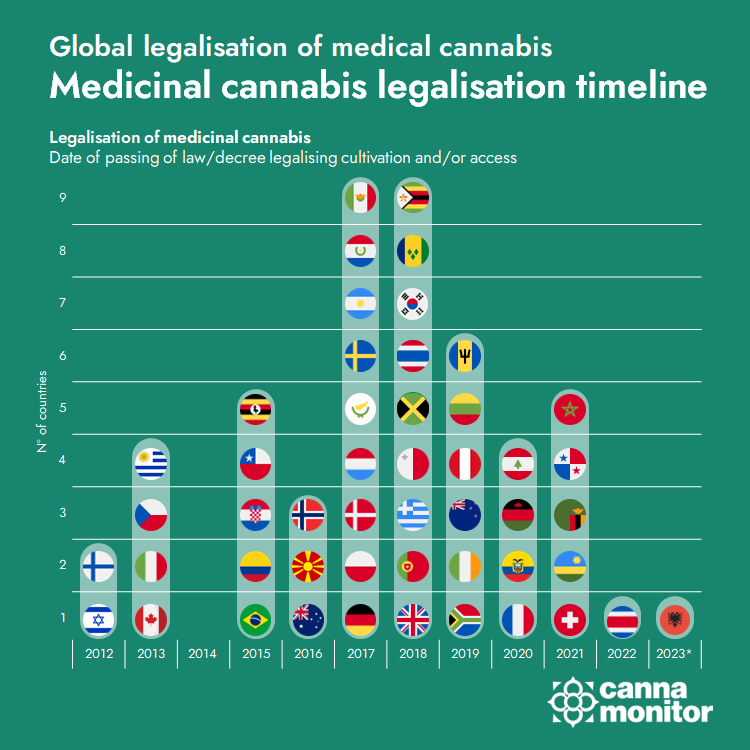
Israel publishes revamped medical cannabis regulations with the potential to bring new momentum to its large medical cannabis market of over 125,000 patients:
- Cannabis will be allowed as first-line treatment and will no longer be considered a ‘last resort’ drug, eliminating the need to try out other therapies.
- Legal CBD without prescription will be allowed, while new modes of administration such as vapes will be admitted in the medical scheme.
- Regulatory burden will be eased: no need to include the exact THC/CBD content in packaging, easier license renewal and removal of price controls.
Poland implements changes to its prescription rules for medical cannabis.
- From now on, a personal examination of the patient must take place every 3 months, either in-person or as part of a teleconsultation.
A new decree by the Italian government includes CBD oral forms as a narcotic, causing uncertainty among the industry in the country:
- This means that CBD oils would only be accessible under a medical prescription at the pharmacy, severely curtailing access to CBD products due to the limited number of prescribers and tight qualifying conditions.
- Currently, a variety of hemp-derived CBD products are available in specialised shops and tobacconists among other retailers. Due to European rules on free movement of goods, imported products will likely remain available.
German adult-use regulations could be further liberalised in the parliamentary process to follow, according to Green and Liberal politicians:
- Deliveries outside association premises and the possibility to sell edibles and drinks are among the reforms that could be introduced to the law.
- The German cabinet has approved its limited plans of legalisation and launched a harm reduction campaign (Legal , but I prefer broccoli ).
Argentina introduced its cannabis and hemp agency ARICCAME, with new production and commercialisation licenses to promote the internal market for industrial hemp derivates, which could create up to 10,000 jobs in the next year.
Legal sales of recreational cannabis have started in Zürich with over 2,000 participants being able to acquire cannabis in 21 different outlets.
- This marks the second pilot project underway after Basel, with other projects planned in Lausanne, Berne, Geneva, Biel, Thun, Olten and Winterthur.
The US Department of Health has sent a letter to the DEA recommending the federal rescheduling of marijuana to recognise its medical use and low potential of abuse:
- This move would open the door to facilitating R&D and allowing the deduction of ordinary business expenses from federal income taxes.
Ontario Cannabis Store is lowering the markups on cannabis products offered to retailers to help with industry profitability.
Infarmed removes CBD cosmetics from the Portuguese market, insisting that CBD cannot be used in such products.
Missouri introduces new rules requiring testing labs to double check each other’s work to prevent increasing concerns of ‘lab shopping’.
The first 2 licences for cannabis consumer associations in Malta have been issued.
Brazil has prohibited the special importation of medicinal cannabis flower in a technical note published by medicinal regulator ANVISA.
-
- The decision was taken following concerns of psychoactive, chemically-modified hemp flower (Delta8-THC) being imported from the US.
- Brazil boasts a booming medicinal CBD market with multiple access pathways: special importation, domestic approval and judiciary authorisations.
2. Medical market entries and CBD exits:
Aurora is winding down its US CBD operations with the closing of Reliva, which it acquired for $40m in 2020.
- This marks the last of many exits by Canadian LPs from the US CBD market, including Canopy, Cronos and The Valens Co, after sales of CBD in the US reportedly peaked in 2021 and have contracted afterwards.
Verdemed, a Canadian pharmaceutical company, has achieved registration for 5 of its CBD medicinal extracts in Brazil, including an equivalent of Epidiolex.
- In a note, Brazil healthcare regulator ANVISA clarifies that only approved products can be advertised in the country, while special imports of mostly CBD products cannot be publicised.
Australian LP Little Green Pharma has achieved registration of a high-THC flower in the Polish medical cannabis market through local partner Medezin.
Organigram, a Canadian LP backed by British American Tobacco announces supply agreement in the UK through 4C labs.
Althea CBD12:THC10 extracts manufactured in Australia will be available to patients in Ireland under its reimbursement scheme.
Tilray consolidates a shift to drinks by taking full ownership of Truss Beverage from Molson Coors, and acquiring 8 beer brands from Anheuser-Busch for $85 million.
Grow Group, a UK-based cannabis company, launches flower strain ‘Euforia’ in the German market through Cantourage.
Mamedica, a medical cannabis clinic, has launched its own-branded products in the UK through a collaboration with Cantourage and Canadian craft grower Miracle Valley.
3. Launch of large observational studies:
LVL Health, a chronic pain clinic in the UK owned by British LP Celadon, has received approval by the Health Research Authority to launch its Canpain clinical study on the use of medical cannabis flower to treat chronic pain among 5,000 patients.
Quest Global Study led by Curtin University and sponsored by Little Green Pharma launches in Australia to assess quality of life and health economic outcomes among medicinal cannabis users.

